Striepen lab Research
We are interested in the cell and molecular biology of protozoan parasites and currently focused on Cryptosporidium, a leading cause of disease in young children. We currently study three specific areas:
The biology of parasite sex
Cryptosporidium has a single host life cycle that relies on both asexual and sexual processes. We found that the parasite follows a hardwired intrinsic program, and that progression to sex is both obligate and essential. The parasite has male and female forms but lacks sex chromosomes or genetic mating loci. We study the epigenetic mechanism that drives parasites to become sexual, and how they 'pick' a specific sex. Male gametes fertilize intracellular females. How do they find each other, and how does the male overcome multiple membrane to deliver its genome? We us a broad range of approaches to discover and dissect molecular mechanism - but are particularly fond of microscopy.

Intracellular parasitism
Cryptosporidium infects the epithelium of the small intestine. Here it invades enterocytes where it establishes and intracellular niche right at the brush border. During and following invasion the parasite secretes a battery of pathogenesis factors from multiple secretion systems. We use a variety of genetic, proteomic, and structural biology approaches to discover these factors and to understand their function in establishing the niche and escaping host responses.
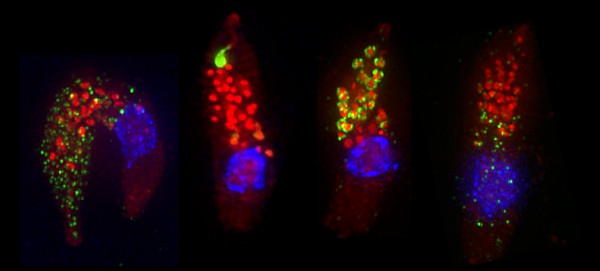
Host parasite interaction and Immunity
Infants are highly susceptible to severe cryptosporidiosis. However, under constant exposure, children older that two rarely show disease because they develop non-sterile disease immunity. A vaccine would be of tremendous benefit, however, the mechanisms that govern host immunity and parasite immune evasion remain largely to be discovered. We have developed facile animal models and reporter parasites to dissect in particular the cellular response to the infection and we are eager to understand how we could induce immunity to protect children. Different parasite strain show marked difference in virulence and persistence and we use genetic crosses between this parasites to discover the genes and mechanisms behind these differences.

